1.Biocomposites, STIMULAN Rapid Cure/Kit Instructions for Use. 2. Cooper, J.J., Method of producing surgical grade calcium sulphate; Patent. 1999. 3. Cooper, J.J., S.S. Aiken, and P.A. Laycock, Antibiotic stability in a synthetic calcium sulphate carrier for local delivery, in 32nd annual meeting of the European Bone and Joint Infection Society. 2013: Prague, Czech Republic. 4. Laycock, P., et al., In Vitro Efficacy of Antibiotics Released from Calcium Sulfate Bone Void Filler Beads. Materials, 2018. 11(11): p. 2265. 5. Delury, C., et al., Determining the Efficacy of Antibiotic-loaded Calcium Sulfate Beads against Pre-Formed Biofilms: An In Vitro Study, in ASM Microbe. 2019: San Francisco, USA. Somasundaram, K., et al., Proximal humeral fractures: the role of calcium sulphate augmentation and extended deltoid splitting approach in internal fixation using locking plates. Injury, 2013. 44(4): p. 481-7. 7. Lei, D., et al., Treatment of Distal Radius Bone Defects with Injectable Calcium Sulphate Cement., in Bone Grafting, A. Zorzi, Editor. 2012, InTech. p. 125-134. 8. Lei, D., L. Jing, and S. Yang-yong, Calcium sulfate versus calcium phosphate in treating traumatic fractures. JOURNAL OF CLINICAL REHABILITATIVE TISSUE ENGINEERING RESEARCH., 2008. 12. 9. Lei, D., Z. Ma, and X. Jing, Treatment of bone defect with injectable calcium sulfate powder in distal fractures of radius. Chinese Journal of Bone Tumor and Bone Disease, 2007. 10. Aiken, S.S., J.J. Cooper, and S. Zhou, Osseointegration of a calcium sulphate bone substitute in a large animal model, in The 5th International Congress of Chinese Orthopaedic Association. 2010: Chengdu, China. 11. Lazarou, S.A., G.B. Contodimos, and I.D. Gkegkes, Correction of alveolar cleft with calcium-based bone substitutes. J Craniofac Surg, 2011. 22(3): p. 854-7. 12. McPherson, E.J., M.V. Dipane, and S.M. Sherif, Dissolvable Antibiotic Beads in Treatment of Periprosthetic Joint Infection and Revision Arthroplasty. The Use of Synthetic Pure Calcium Sulfate (Stimulan®)Impregnated with Vancomycin & Tobramycin. Reconstructive Review, 2013. 3(1): p. 32-43. 13. McPherson, E.J., Dissolvable Antibiotic Beads in Treatment of Periprosthetic Joint Infection. The Use of Synthetic Pure Calcium Sulfate (Stimulan®) Impregnated with Vancomycin & Tobramycin, in 2nd Annual Oxford Bone Infection Conference (OBIC). 2012: Oxford, UK. 14. Gauland, C., The use of antibiotic impregnated, implanted synthetic calcium sulphate tablets in the treatment of soft tissue, vancomycin resistant, enterococcus infections, in The Symposium on Advanced Wound Care and Wound Healing Society Annual Meeting. 2011: Gaylord Texan Hotel & Convention Center, Dallas, Texas. 15. Borrelli, J., Jr., W.D. Prickett, and W.M. Ricci, Treatment of nonunions and osseous defects with bone graft and calcium sulfate. Clin Orthop Relat Res, 2003(411): p. 245-54. 16. Helgeson, M.D., et al., Antibiotic-impregnated calcium sulfate use in combat-related open fractures. Orthopedics, 2009. 32(5): p. 323. McKee, M.D., et al., The use of an antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute in the treatment of infected long bone defects: early results of a prospective trial. J Orthop Trauma, 2002. 16(9): p. 622-7. 18. Noor, S., et al., The use of Osteoset-T in the treatment of chronic osteomyelitis of the tibia following exogenous trauma: A review of 22 patients at a regional trauma centre. Bone & Joint Journal Orthopaedic Proceedings Supplement, 2013. 95-B(SUPP 23): p. 4. 19. Analysis of the Wear Effect 3rd Body Particulate (Bone Cement) has on UHMWPE, Accutek Testing Laboratory, Fairfield OH, K13107732-1, 2014. 20. Cowie, R.M., et al., The influence of a calcium sulphate bone void filler on the third-body damage and polyethylene wear of total knee arthroplasty. Bone Joint Res, 2019. 8(2): p. 65-72.
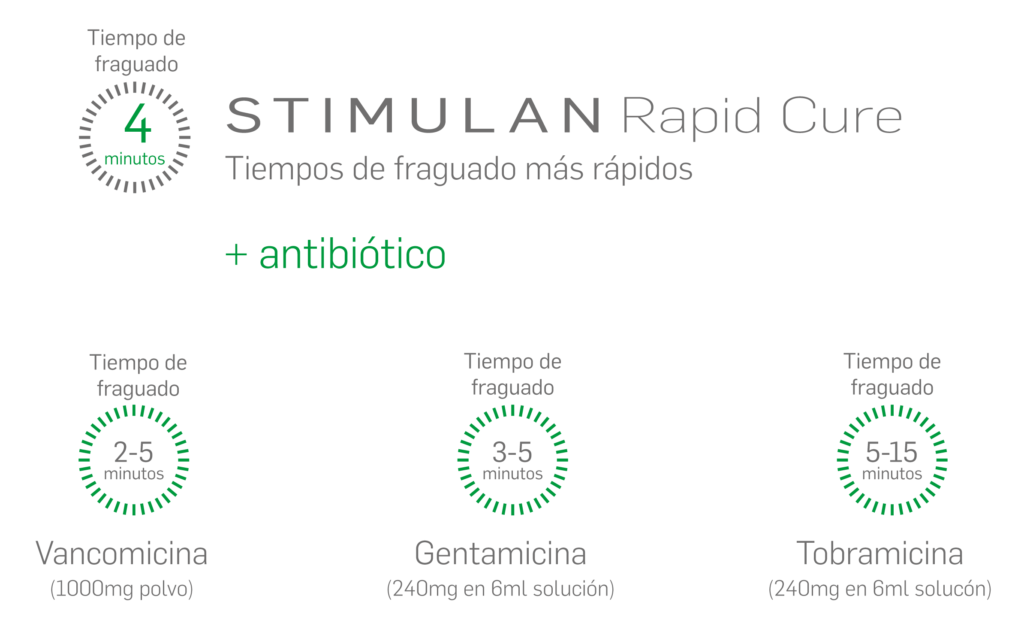
Siga las indicaciones y consulte las contraindicaciones, advertencias y precauciones en las instrucciones de uso. Es responsabilidad del especialista decidir el tipo y la cantidad de antibiótico empleado. El uso simultáneo de antibióticos administrados localmente podría afectar al tiempo de fraguado.
La práctica de mezclado de antibióticos con el dispositivo STIMULAN Kit / STIMULAN Rapid Cure no ha sido evaluada por ninguna autoridad competente europea de medicamentos y está considerada como una indicación no autorizada conforme a las especificaciones del medicamento. Realizar esta práctica corre por cuenta y riesgo del cirujano / profesional sanitario.
Este catálogo puede contener el uso de STIMULAN y/o técnicas que van más allá de la autorización / aprobación actual concedida por la autoridad reguladora competente. Para obtener más información, póngase en contacto con su representante local.
©2019, Biocomposites, STIMULAN, Bringing Calcium to Life, Power to Transform Outcomes y Dry26 son marcas / marcas registradas de Biocomposites Ltd. Todos los derechos reservados. No está permitida la copia, reproducción, distribución o reedición sin el permiso expreso y por escrito del propietario, Biocomposites Ltd.
Patentes concedidas: GB2367552, EP 1204599 B1, US 6780391, EP 2594231 B1, US 8883063, CN ZL201210466117.X, GB2496710, EP 3058899 B1 / Patentes pendientes: GB1502655.2, US 15/040075, CN 201610089710.5, US 15/288328, GB1704688.9, EP 18275044.8, US 15/933936, CN 108619579A
MA0048R3/MA0252R1/MA0093R3/MA0238R1