References and regulatory statements
Biocomposites GmbH (former ARTOSS GmbH)
- Kienast B et al. (2016). Nanostrukturiertes synthetisches Knochenersatzmaterial zur Behandlung von Knochendefekten. Trauma und
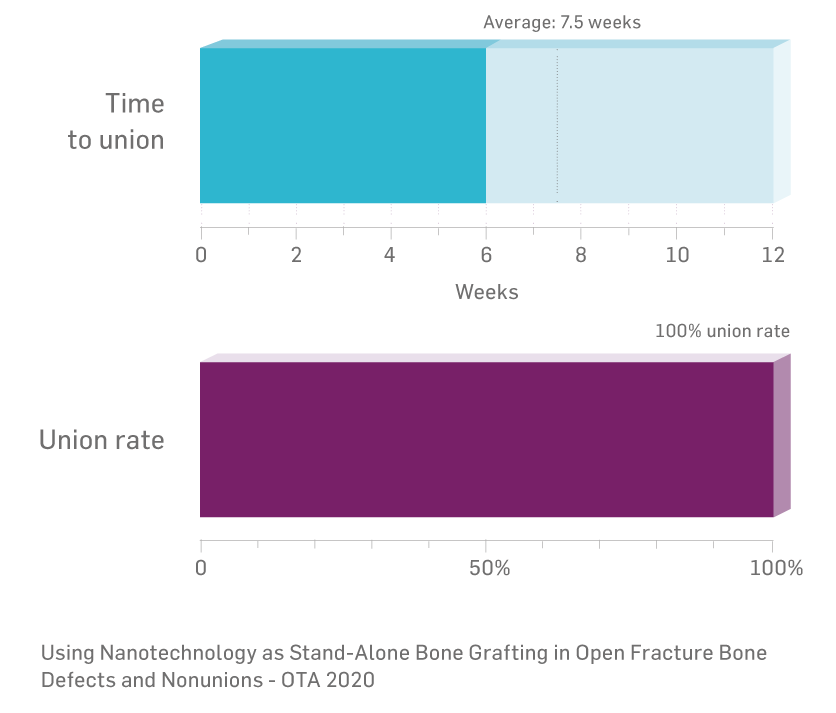
Berufskrankheit, 4(18), 308-18. - Ortega, G. Using Nanotechnology as stand-alone bone grafting in open fracture bone defects and nonunions, Orthopaedic Trauma Association Annual Meeting, #1043, 2020.
- Xu W (2011). Evaluation of injectable silica-embedded nanohydroxyapatite bone substitute in a rat tibia defect model. Int J Nanomedicine, 6, 1543-52.
- NanoBone® Summary of product characteristics.
- Meier J et al. (2008). Application of the synthetic nanostructured bone grafting material NanoBone® in sinus floor elevation. Implantologie, 16, 301-14.
- Fratzl P et al. (1991). Nucleation and Growth of Mineral Crystals in Bone Studied by Small-Angle-X-Ray Scattering. Calcif Tissue Int, 48, 407-413.
- Weiner S et al. (1986). Disaggregation of Bone Into Crystals. Calcif Tissue Int, 39, 365-375.
- Scherrer P., (1918). Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen, Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen, Mathematisch-Physikalische Klasse, 98-100.
- Kirchhoff M et al. (2011). Lateral augmentation of the mandible in minipigs with a synthetic nanostructured hydroxyapatite block. Journal of Biomedical Materials Research. Part B, Applied Biomaterials, 96(2), 342–350.
- Götz W et al. (2008). Immunohistochemical characterization of nanocrystalline hydroxyapatite silica gel (NanoBone) osteogenesis: a study on biopsies from human jaws. Clinical Oral Implants Research, 19(10), 1016–1026.
- Gerber T et al. (2012). Nanostructured bone grafting substitutes–A pathway to osteoinductivity. In Key Engineering Materials, 493,
147-152. - Data on file, External testing: Specific surface area, 2010.
- Data on file.
- Abshagen K et al. (2009). In vivo analysis of biocompatibility and vascularization of the synthetic bone grafting substitute NanoBone®. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 91(2), 557-566.
- Rosenthal H (2022). Evaluating a Nanocrystalline Hydroxyapatite Bone Graft Substitute for the Treatment of Benign Bone Tumors. The Internet Journal of Orthopedic Surgery, 30(1).
©2024, Biocomposites is a trademark/registered trademark of Biocomposites Ltd. NanoBone is a trademark/registered trademark of Biocomposites GmbH. All rights reserved. No unauthorized copying, reproduction, distributing or republication is allowed unless prior written permission is granted by the owner, Biocomposites Ltd.
Patents granted: EP 1 624 904 B1, US 8,715,744 B2, JP4764821B2, 284158, CA2537620C, RU2354408C2, ZL200480020915.3, DE 50 2004 002 554.4, ES2280969T3, AU 2004241740 B2, HK1080766A1, EP 3 600 464 B1, US 11,324,859 B2, JP7118132B2, CN110650754B, DE 50 2018 009 567.7, ES2917406T3, MX2019011659A, RU2768695C2, 386769, AU2018246310A1, BR112019020029A2, CA3058253A1
MA0450R3