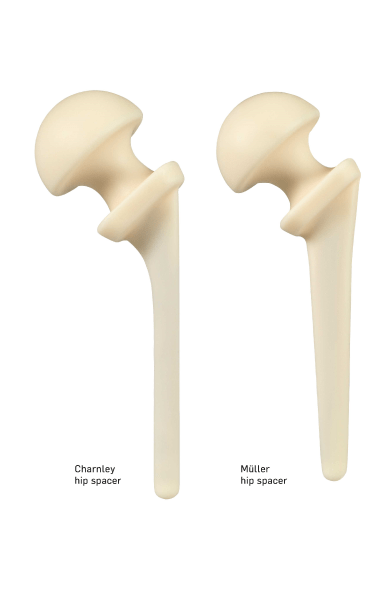
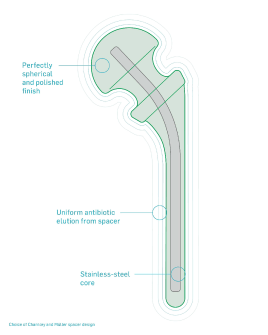
References and regulatory statements
- Craig, A. et al., (2022). Articular spacers in two-stage revision arthroplasty for prosthetic joint infection of the hip and the knee. EFORT Open Reviews, 7, 137–152.
- Ocguder, A. et al., (2010). Two-stage total infected knee arthroplasty treatment with articulating cement spacer. Archives of orthopaedic and trauma surgery, 130(6), 719–725.
- Dunne, N. et al., (2007). In vitro study of the efficacy of acrylic bone cement loaded with supplementary amounts of gentamicin: effect on mechanical properties, antibiotic release, and biofilm formation. Acta orthopaedica, 78(6), 774–785.
- Hollyer, I. et al., (2023). Selecting a high-dose antibiotic-laden cement knee spacer. Journal of orthopaedic research : official publication of the Orthopaedic Research Society, 41(7), 1383–1396.
- Moerenhout, K. et al., (2021). Economic advantage of ‘self-made’ antibiotic-loaded spacer compared to prefabricated antibiotic-loaded spacer and spacer molds in two-staged revision arthroplasty. Acta orthopaedica Belgica, 87(3), 557–562.
- SYNICEM Spacers: Instructions for Use.
- Biocomposites, Data on file.
©2025, Biocomposites, STIMULAN are trademarks/registered trademarks of Biocomposites Ltd. Synimed and Synicem are trademarks/registered trademarks of Synergie Ingénierie Médicale S.A.R.L. All rights reserved. No unauthorized copying, reproduction, distributing or republication is allowed unless prior written permission is granted by the owner, Biocomposites Ltd.
Patents granted: GB2367552, EP 1204599 B1, US 6780391, EP 2594231 B1, US 8883063, CN ZL201210466117.X, GB2496710, EP 3058899 B1, US 10390954, US 10,588,748, CN ZL201610089710.5
Patents pending: GB1502655.2, GB1704688.9, EP 18275044.8, US 15/933936, CN 108619579A
MA0500R1