Sports injuries
Arthroscopic procedures and the use of absorbable implants are common for treating sports injuries, as they provide a secure graft fixation while allowing biocompatible absorption and eventual replacement by bone.
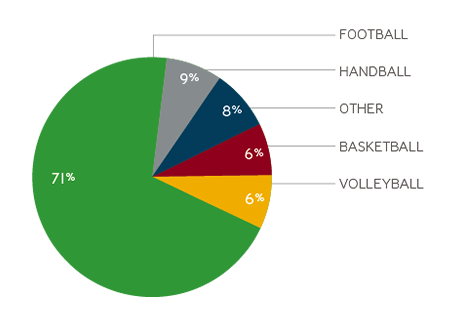
In the EU, sports injuries accounts for around 6.2 million people annually needing hospital treatment; 7% (402,000 cases) having to be admitted for further treatment.1
- Team ball sports account for 42% of all hospital-treated sports injuries, with 71% of these linked to football.1
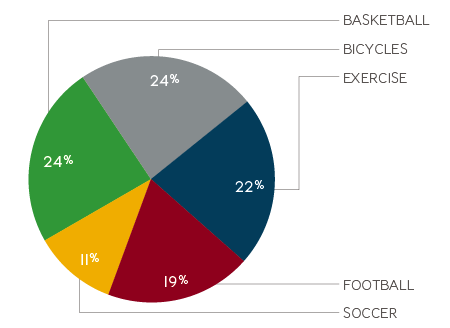
In the USA, it is estimated that 30 million children and adolescents participate in youth sport. High school athletes account for an estimated 2 million injuries, 500,000 doctors’ visits and 30,000 hospitalisations each year.2,3
- Children between the ages of 5-14 account for an estimated 40% of all sports-related injuries treated in hospitals.2,4
Percentage of people treated in EU hospitals for sports injuries each year1
Number of people treated in US hospitals for top 5 sports injuries5
Anterior Cruciate Ligament (ACL)
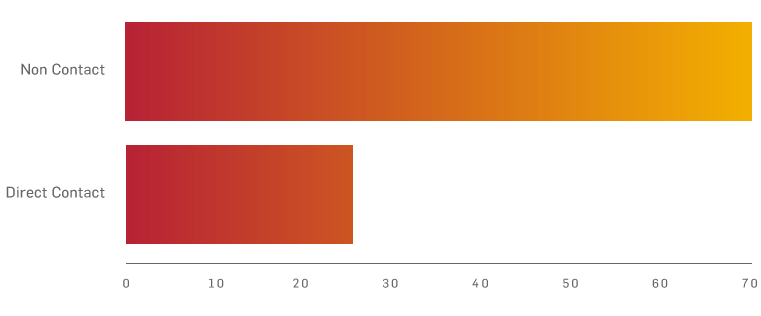
There are more than 200,000 ACL injuries each year in the USA, with approximately half of them requiring knee reconstruction. Around 70% occur while playing agility sports such as basketball, soccer, skiing and football. Within this number, 70% are sustained through non-contact mechanisms and 30% from direct contact.6,7,8
- In the USA, the estimated cost of treatment for ACL injured patients is over $1.7 billion annually.9
- In the USA, the estimated cost per patient for ACL surgery and rehabilitation is between $17,000 and $25,000.9
Estimated cause of ACL injuries7
For many years, absorbable implants have been used by surgeons in preference to metal devices as they offer a number of advantages:
- No distortion of MRI imaging
- No risk of metal contamination
- No need for a second surgical procedure to remove the implant
There is now a wide range of absorbable devices. Some of these include absorbable polymer implants which are presenting problems associated with extended periods of absorption, migration, strength properties and foreign body reactions.
Biocomposites has pioneered the development of calcium polymer composite materials with improved mechanical properties, which give support during the healing period, offer improved biocompatibility during absorption and allow healing by bony growth.