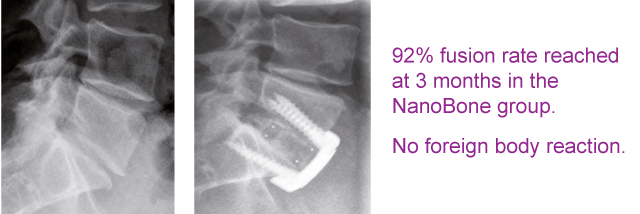
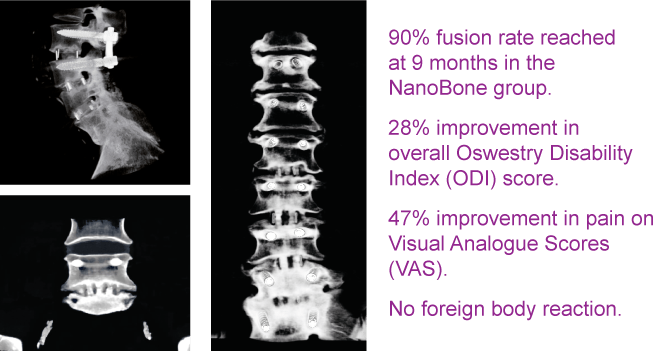
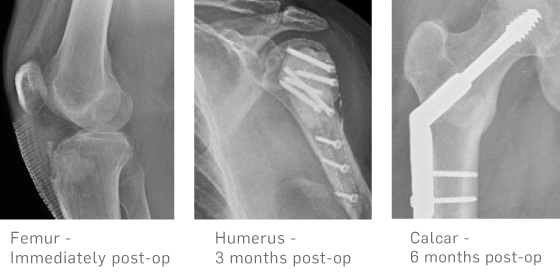
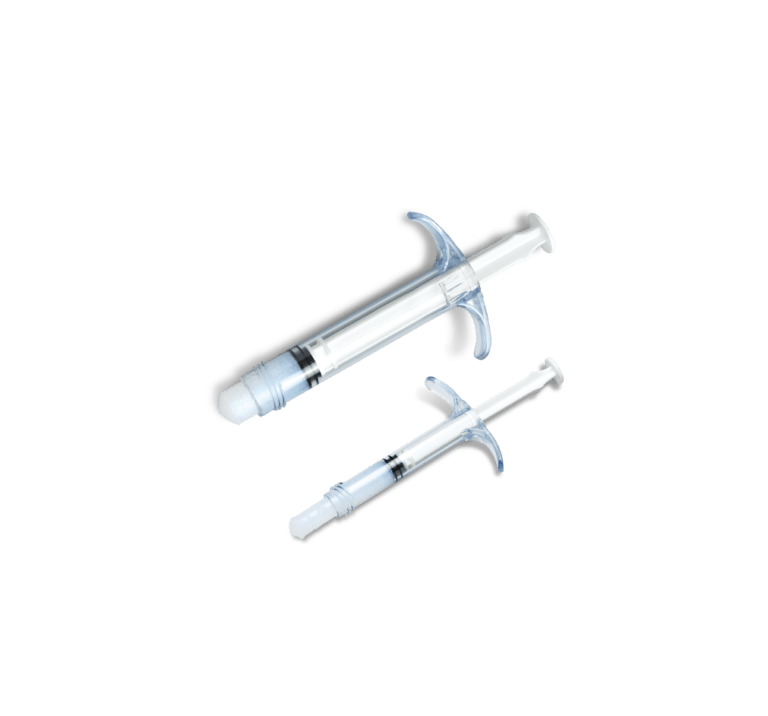
Biocomposites GmbH (former ARTOSS GmbH)
1. Kienast B et al. (2016). Nanostrukturiertes synthetisches Knochenersatzmaterial zur Behandlung von Knochendefekten, Trauma und
Berufskrankheit, 4(18), 308-18. 2. Götz W et al. (2010). A preliminary study in osteoinduction by a nano-crystalline hydroxyapatite in the mini pig. Folia histochemica et cytobiologica, 48(4), 589-596. 3. Xu W (2011). Evaluation of injectable silica-embedded nanohydroxyapatite bone substitute in a rat tibia defect model. Int J Nanomedicine, 6, 1543-52. 4. NanoBone® Summary of product characteristics. 5. Meier J et al. (2008). Application of the synthetic nanostructured bone grafting material NanoBone® in sinus floor elevation, Implantologie, 16, 301-14. 6. Fratzl, P., et al., (1991). Nucleation and Growth of Mineral Crystals in Bone Studied by Small-Angle-X-Ray Scattering, Calcif Tissue Int., 48, 407-413. 7. Weiner, S., et al., (1986). Disaggregation of Bone Into Crystals, Calcif Tissue Int., 39, 365-375. 8. Scherrer P., (1918). Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen, Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen, Mathematisch-Physikalische Klasse, 98-100. 9. Kirchhoff M et al. (2011). Lateral augmentation of the mandible in minipigs with a synthetic nanostructured hydroxyapatite block. Journal of biomedical materials research. Part B, Applied biomaterials, 96(2), 342–350. 10. Götz W et al. (2008). Immunohistochemical characterization of nanocrystalline hydroxyapatite silica gel (NanoBone) osteogenesis: a study on biopsies from human jaws. Clinical oral implants research, 19(10), 1016–1026. 11. Gerber T et al. (2012). Nanostructured bone grafting substitutes – A pathway to osteoinductivity. In Key Engineering Materials, 493, 147-152. 12. Data on file, External testing: Specific surface area, 2010. 13. Abshagen K et al. (2009). In vivo analysis of biocompatibility and vascularization of the synthetic bone grafting substitute NanoBone®. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 91(2), 557-566. 14. Rickert M et al. (2019). Clinical Outcome After Anterior Lumbar Interbody Fusion with a New Osteoinductive Bone Substitute Material: A Randomized Clinical Pilot Study. Clinical spine surgery, 32(7), E319–E325. 15. Hebecker R et al. (2008, June 1). A new nanostructured bone substitute for use in neurosurgery – results of a prospective study in lumbar fusion and further applications. 59th Annual Meeting of the German Society of Neurosurgery (DGNC) 3rd Joint Meeting with the Italian Neurosurgical Society (SINch). 16. Rosenthal H. (2022). Evaluating a Nanocrystalline Hydroxyapatite Bone Graft Substitute for the Treatment of Benign Bone Tumors. The Internet Journal of Orthopedic Surgery, 30(1).
©2024, Biocomposites is a trademark/registered trademark of Biocomposites Ltd. NanoBone is a trademark/registered trademark of Biocomposites GmbH. All rights reserved.
No unauthorised copying, reproduction, distributing or republication is allowed unless prior written permission is granted by the owner, Biocomposites Ltd.
Patents granted: EP 1 624 904 B1, US 8,715,744 B2, JP4764821B2, 284158, CA2537620C, RU2354408C2, ZL200480020915.3, DE 50 2004 002 554.4, ES2280969T3, AU 2004241740 B2, HK1080766A1, EP 3 600 464 B1, US 11,324,859 B2, JP7118132B2, CN110650754B, DE 50 2018 009 567.7, ES2917406T3, MX2019011659A, RU2768695C2, 386769, AU2018246310A1, BR112019020029A2, CA3058253A1
MA0443R2