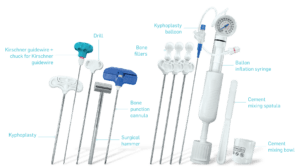
References and regulatory statements
References: 1. Surgical cement for Kyphoplasty, Instructions for Use. 2. Sterile balloon Kyphoplasty set, Instructions for Use.
©2025, Biocomposites is a trademark/registered trademark of Biocomposites Ltd. SYNICEM is a trademark/registered trademark of Biocomposites International Ltd. All rights reserved. No unauthorised copying, reproduction, distributing, or republication is allowed unless prior written permission is granted by the owner, Biocomposites Ltd/Biocomposites International Ltd.
SYNICEM KYP and SYNICEM KYP SYSTÈME are manufactured by Synergie Ingénierie Médicale S.A.R.L.
Synergie Ingénierie Médicale S.A.R.L. (Synimed), Zone Artisanale de l’Angle, 19370 Chamberet, France.
MA0525R1